Our system integrates a proven boron source, boronophenylalanine (BPA) along with a reliable cancer-targeting agent (folic acid) into a multifunctional, layered gold nanoparticle (LGNP). This is done by starting with a 5 nm gold core, after which alternating layers of cationic polyelectrolyte (4-Borono-L-Phenylalanine-Poly(fluorescein isothiocyanate allylamine) hydrochloride (BPA:FITC-PAH)) and anionic polyelectrolyte (4-Borono-L-Phenylalanine-Poly(4-styrene sulfonate) (BPA:PSS)) are electrostatically deposited onto the nanoparticle surface. Lastly, folic acid is electrostatically functionalized to the surface of the layered particle. This layered gold nanoparticle forms the basis of the Boron Neutron Capture Therapy segment of the project.
To create a more versatile and all-around complete system, the LGNP was combined with tensegrity DNA origami structures. These tensegrity DNA origami structures are synthesized, and then "walked" over a 150 nm by 100 nm flat DNA origami tile. These "walkers" have three binding sites to which cargoes may be attached during walking. The LGNP is bound to one of these sites via EDC-sulfo-NHS coupling (see protocols). This leaves two unoccupied sites for other cargoes to add further capabilities to our system, such as tracking and imaging, additional drug delivery or enhanced targeting.
We initially explored several options for these extra cargoes, including quantum dots and iron oxide nanoparticles for enhanced imaging. Though the gold nanoparticle is a capable MRI enhancer, additional imaging and tracking would be valuable to the system. We then realized that it would be necessary to consider the size of our LGNP relative to the walker and tile, as well as the possible consequences of adding another larger cargo. Due to limited time and resources, we were unable to test these in the lab over the summer.
Despite this, we were able to utilize one of the two available binding sites with biotinylated DNA. This was used as a proof-of-concept for a true cancer-targeting agent; in a clinical setting, a cancer-targeting agent would be substituted. The key accomplishment here is that we were able to demonstrate the possibility of adding a second useful cargo to our LGNP-walker system.
In summary, we demonstrate a fully integrated, self-assembling DNA origami-gold nanoparticle cancer-targeting system based on Boron Neutron Capture Therapy (BNCT) principles. The system brings together a BPA and folic acid functionalized layered gold nanoparticle for BNCT with tensegrity DNA origami walkers for the possible addition of further capabilities and applications with more cargoes. Though more work would be required to investigate these additional applications further, it was possible to conjugate biotinylated DNA to our walkers, thereby indicating the possibility of adding a cancer-targeting agent. Nevertheless, the system as it is actively targets and destroys cancer cells while leaving healthy cells unharmed.
Boron Neutron Capture Therapy (BNCT) is a promising alternative treatment for head and neck cancers. Initially proposed as a possible cancer treatment in 1936 by Gordon L. Locher, BNCT has been previously investigated by various research groups [4]. BNCT is an attractive and strongly pursued cancer treatment due to its ability to selectively target and destroy tumour cells, which is beyond the capability of traditional cancer treatments including chemotherapy.
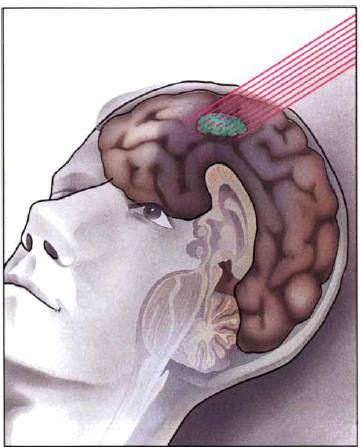
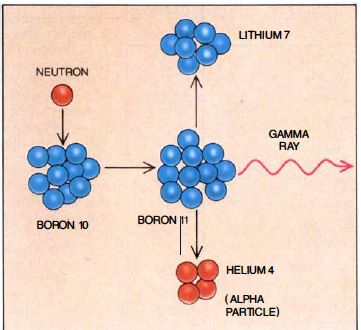
BNCT is based on a simple reaction involving neutrons and boron-10. When low- energy neutrons come into contact with boron-10, the unstable boron-11 isotope forms. The isotope then undergoes nuclear fission, resulting in high-energy lithium-7 nuclei (or lithium 3+ ions) and alpha particles carrying 2.31 MeV of energy as shown below.
$$_10^5B \text" "+ \text" " n \html" → "_11^5B \html" → " Li^{3+} \text" " + \text" " He^{2+} \text" "+ \text" " 2.31 \text" "MeV$$
This alpha-particle radiation will destroy any biological material within a 5-9 μm radius of the boron source, roughly the diameter of a cell. In principle, if a sufficient amount of boron could be accumulated in tumour cells with negligible uptake into healthy cells, this would prove to be a viable targeted cancer therapeutic system.
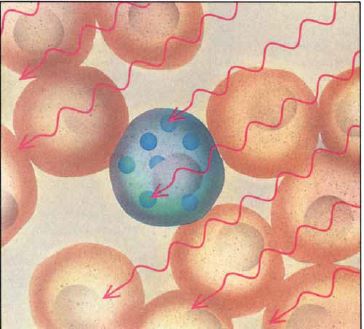
There are two challenges imposed with this method: First the need to target the tumour cells for selective delivery of the boron source, and second, to ensure that a sufficient quantity of boron is up-taken for guaranteed cell death.
Multiple approaches to these problems have been explored in recent years. This includes the boron source used in this project, boronophenylalanine (BPA), which has been used in clinical trials in both Finland and Sweden from 1999-2002 [5,6]. To address the targeting issue, folic acid is used as a targeting agent as it will preferentially attach to the over-expressed folic acid receptors on the tumour cells.